Learn how ammonia emissions negatively impact pig performance and how to mitigate them
The most common complaint producers hear when people drive past or visit a pig barn is the smell. Ammonia, a gas released when manure decomposes, can be easily recognized by its pungent odor. Ammonia can be detrimental to the health and welfare of animals and employees, when at low concentrations. Livestock production is responsible for almost 64% (Dopelt et al., 2019) of global ammonia emissions. The global swine industry is responsible for about 15% of ammonia emissions associated with livestock. The swine industry’s contribution varies by region due to the concentration of animals and can be as high as 60% in areas of China and 25% in Europe (Olivier et al., 1998; Philippe et al., 2011; Xu et al., 2014).
Considering the scenario, many swine producers must ask: how can we control ammonia in grow-finish operations? First, it’s necessary to understand where ammonia originates. In swine operations, ammonia is a byproduct of microbial decomposition of urine and feces in the manure storage pit below the floor.
A simple explanation for this complex process is:
- Urea, from urine, is converted to ammonia and carbon dioxide by the microbial enzyme urease (from microbes excreted in feces).
- Once formed, free ammonia can take two forms, depending on the pH of the manure: NH3 (ammonia) or the ammonium ion (NH4+)
- At low pH, most of the ammonia remains in the liquid as NH4+
- At pH greater than 7, the ammonium ion is converted to ammonia (NH3) and can escape as gas

Strategies to control ammonia production
Ammonia volatilization is a process that depends on many factors such as relative humidity, animal density and activity, amount of manure and urine on the floor, airspeed in the building, and dry matter content in the manure (Blanes-Vidal et al., 2008; Fabbri et al., 2007). Throughout all stages of pig production, ammonia production is a concern, but most specifically in grow-finish operations, which account for 60-70% of the total nitrogen excretion (Jongbloed and Lenis, 1993). Considering the above, efforts to reduce ammonia production are necessary.
Environmental and nutritional management practices are necessary to reduce ammonia levels in pig production significantly. Environment control, especially ventilation, is one strategy to reduce ammonia emissions. According to Tabase et al. (2018), managing the NH3 emission from livestock buildings requires introducing fresh air into the units while avoiding over-ventilation above the manure-covered surfaces. Another way of introducing fresh air is to adjust inlet openings, promoting constant airflow into the building. Biofilters and scrubbers can also be used in mechanically ventilated systems, as they encourage air purification.
Manure management is also crucial when it comes to reducing ammonia emissions. Removal of manure is recommended 1-2 times per day from pig stalls. Storing manure for an extended period increases ammonia production within a facility. For example, a storage period of 3 days can result in a 40% increase in NH3 (Botermans et al., 2010) Therefore, under ideal circumstances emptying the pit frequently is advised. Additionally, methods for reducing microbial activity in manure may be helpful, such as using additives to lower the pH.
Pen design and hygiene are also essential factors to consider. Cleaning the slats the lying area should be the primary objective. Botermans et al. (2010) suggested a slope with an incline between 2% and 3% on the floor to help drain uring. Furthermore, the excretion area should account for at least 25% of the lying area. Keeping pigs clean and dry is also critical, especially in the summer, when heat-stressed animals change their behavior and start lying on the slatted floor.
Dietary manipulation can also aid in the control of ammonia emissions by minimizing the crude protein level within the diet. Le et al. (2009) demonstrated that reducing crude protein in pig diets can significantly reduce ammonia emission from manure. Portejoie et al (2014) reported that dietary protein reduction from 20% to 12% resulted in a 63% reduction of ammonia emissions.
How does ammonia affect grow-finish operations without proper control?
The negative impact of ammonia emissions goes far beyond its irritating odor. High concentrations of ammonia are detrimental to the health of both pigs and the people working in the facilities. Ammonia levels of 7 ppm can reduce pulmonary function in swine farm workers (Donham et al., 1989; Donham et al., 1995), and concentrations above 35 ppm promote inflammatory changes in the wall of the respiratory tract and reduce bacterial clearance from lungs in young pigs (Drummond et al., 1978). High levels of ammonia within the barn can also cause an increase in pig restless, and ear, tail, and flank biting. In addition, ammonia concentrations of 50 ppm or above can cause inflammation of the respiratory tract, increasing susceptibility to respiratory infections by reducing the rate of bacterial clearance (Gustin et al., 1994; Urbain et al., 1994). High levels also affect animal performance, reducing growth rates up to 12% during prolonged periods of exposure (Drummond et al., 1980).
Conclusion
Ammonia emission is a constant challenge for all swine producers since high concentrations of this gas have harmful effects on pig health and performance. This article has provided an overview of ammonia volatilization, how grow-finish pigs are affected, and practical strategies for reducing its concentration in swine building. If a single ammonia mitigation method doesn’t work, farms should consider using several approaches to control ammonia in grom-finishing operations.
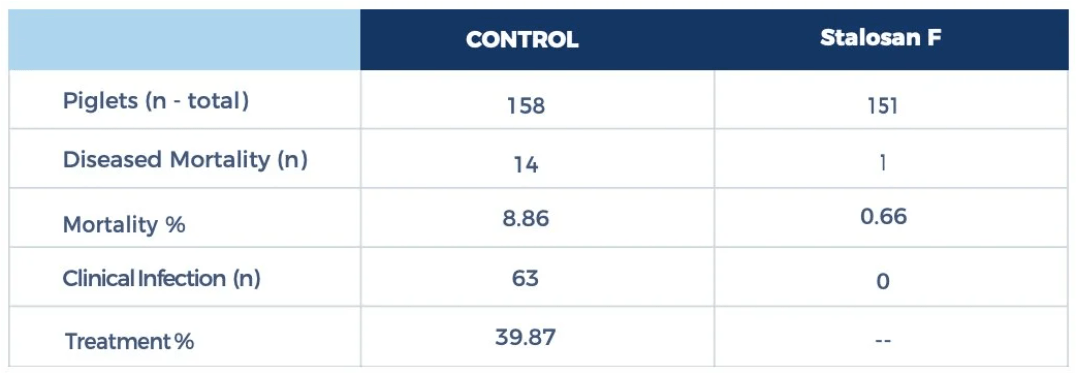
Find more information at protekta.com for ammonia mitigation solutions, including Stalosan F: a valuable tool when it comes to ammonia control. Stalosan F acts as a buffer that chemically controls manure moisture and pH. Furthermore, Stalsoan F inhibits urease enzyme activity, decreasing the conversion of urea to ammonia.
References
Blanes-Vidal, V., Hansen, M. N., Pedersen, S., & Rom, H.B. (2008). Emissions of ammonia, methane and nitrous oxide from pig houses and slurry: Effects of rooting material, animal activity, and ventilation floe. Agriculture, Ecosystems, & Environment, 124(3-4), 237-244. DOI: https://doi.org/10/1016/
j.agee.2007.10.002 Botermans, J., Gustafsson, G., Jeppsson, K.H., Brown, N., & Rodhe, L. (2010). Measures to reduce ammonia emissions in pig production (No. 2010: 1). Donham, K., Haglind, P., Peterson, Y., Rylander, R., & Belin, L. (1989). Environmental and health studies of farm workers in Swedish swine confinement buildings. Occupational and Environmental Medicine, 46(1), 31-37. Donham, K. J., Reynolds, S. J., Whitten, P., Merchant, J.A., Burmerister, L., & Popendorf, W.J. (1995). Respiratory dysfunction in swine production facility workers: Dose-response relationships of environmental exposures and pulmonary function. American Journal of Industrial Medicine, 27(3), 405-418. DOI: 10.1002/ajim.4700270309 Dopelt, K., Radon, P., & Davidovitch, N. (2019), Environmental effects of livestock industry: the relationship between knowledge, attitudes, and behavior among students in Isreal. International Journal of Environmental Research and Public Health, 16(8), 1359. DOI: https://doi.org/10.3390/
ijerph16081359 Drummonds, J. G., Curtis, S. E., Simon, J., & Norton, H. W. (1980). Effects of aerial ammonia on growth and health of young pigs. Journal of Animal Science, 50(6), 1085-1091. https://doi.org/10.2527/
jas1980.5061085x Drummonds, J. G., Curtis, S. E., Simon, J. (1978). Effects of atmospheric ammonia on pulmonary bacterial clearance in the young pig. American Journal of Veterinary Research, 39(2), 211-212. Fabbri, C., Valli, L., Guarino, M., Costa, A., & Mazzotta, V. (2007). Ammonia, methane, nitrous oxide, and particulate matter emissions from two different buildings for laying hens. Biosystems Engineering, 97(4), 441-455. DOI: 10.1016/j.biosystemseng.2007
.03.036 Gustin, P., Urbain, B. R. U. N. O., Prouvost, J. F., & Ansay, M. (1994). Effects of atmospheric ammonia on pulmonary hemodynamics and vascular permeability in pigs: interaction with endotoxins. Toxicology and Applied Pharmacology, 125(1), 17-26. DOI: 10.1006/taap.1994.1044 Jongbloed, A. W. and N. P. Lenis (1993). Excretion of nitrogen and some minerals by livestock. In: M.W.A. Verstegen, L.A. Den Harog, G.J.M. van Kempen, and J.H.M. Metx (Eds). Nitrogen Flow in Pig Production and Environmental Consequences. EAAP Publ. Pudoc, Wageningen, The Netherlands. 69:22-36. Le, P.D., Aarnink, A. J. A., & Jonjbloed, A. W. (2009). Odor and ammonia emissions from pig manure as affected by dietary crude protein level. Livestock Science, 121(2-3), 267-274. DOI: https://doi.org/10.1016/j.livsci
.2008.06.021 Olivier, J. G. J., Bouwma, A. F., Van der Hoek, K. W., & Berdowski, J. J. M. (1998). Global air emission inventories for anthropogenic sources of NOx, NH3, and N2O in 1990. Environmental Pollution, 102(1), 135-148. DOI: https://doi.org/10.1016/S0269-7491(98)80026-2 Philippe, F. X., Cabaraux, J. F., & Nicks, B. (2011). Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agriculture, Ecosystems & Environment, 141(3-4), 245-260. DOI: https://doi.org/10.1016
/j.agee.2011.03.012 Portejoie, S., Dourmad, J. Y., Martinez, J., & Lebreton, Y. (2004). Effect of lowering dietary crude protein on nitrogen excretion, manure composition, and ammonia emission from fattening pigs. Livestock Production Science, 91(1-2), 45-55. https://doi.org/10.1016
/j.livprodsci.2004.06.013 Tabase, R. K., Millet, S., Brusselman, E., Ampe, B., Sonck, B., & Demeyer, P. (2008). Effect of ventilation setting on ammonia emission in an experimental pig house equipped with artificial pigs. Biosystems Engineering, 176, 125-139. DOI: https://doi.org/10.1016
/j.biosystemseng.2018.10.010. Urbain, B., Gustin, P., Prouvost, J. F., & Ansay, M. (1994). Quantitive assessment of aerial ammonia toxicity to the nasal mucosa by use of the nasal lavage method in pigs. American Journal of Veterinary Research, 55(9), 1335-1340. Xu, W., Zheng, K., Liu, X., Meng, L., Huaitialla, R. M., Shen., … & Zhang, F. (2014). Atmospheric NH3 dynamics at a typical pig farm in China and their implications. Atmospheric Pollution Research, 5(3), 455-463. DOI: https://doi.org/10.5094
/APR.2014.053